Novum Canada is a one stop solution to all your regulatory bioanalytical needs supporting the development of pharmaceuticals and biosimilars. With over 15 years of extensive experience, our scientists will partner with your team to develop robust assays for parent drug and metabolites. The team will develop robust and reproducible methods meeting your sponsor and regulatory requirements. Our commitment and focus to quality are reflected through our processes and systems.
Novum Canada has been involved in conducting over 400 assays involving small and large molecules supporting pharmacokinetic, toxicokinetic, studies in our state of art GLP compliant facility. Our scientists routinely develop and validate methods and analyze over 15000 samples a month. We have developed complex to proprietary methods, for generic and NCE molecules, which include sub picogram detection, endogenous, chiral and hormonal assays. We use state of the art automation systems in our lab including EDC and reporting process.
Our team will work closely with you and provide quick turnaround time ensuring operational efficiencies and quality of data.
Core expertise includes developing and validating methods that are robust and reproducible, and employing these methods in a high throughput environment to accelerate development times for prescription medicines (Rx drugs) and generics.
With state-of-the-art instrumentation, we are one of the leading bioanalytical providers in the industry. We strive to deliver data which exceeds quality standards and will stand scrutiny to regulatory requirements
Bioanalytical Services:
- Method Development
- Method Validation
- Pharmacokinetic (PK) Analysis
- Bioavailability Studies
- Bioequivalence Studies
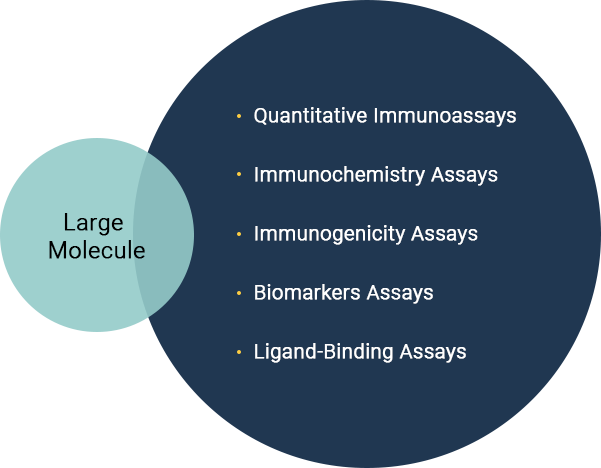
- Large Molecule analysis
- Short and long term sample storage with an online monitoring and warning system
BIOANALYTICAL METHODS – assay list
The list covers –
- Recently Validated Methods
- Premium Methods
- Large Molecules / Therapeutic Proteins / Biosimilars
- Immunogenicity Assessment
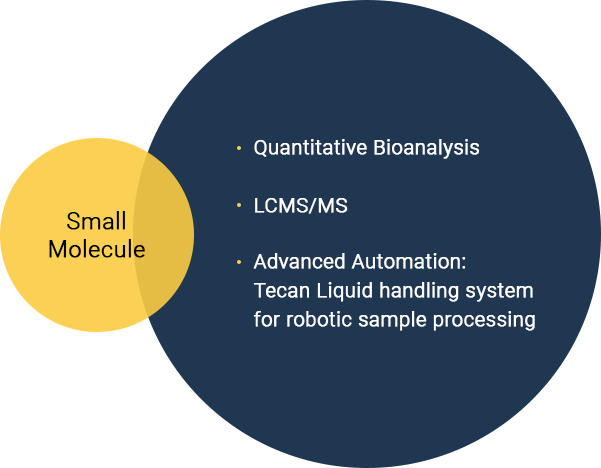
- Small Molecules
- New Chemical Entities
- In-Vitro Studies
- PD Markers
- Healthcare Hygiene Products