Experience/Therapeutic areas
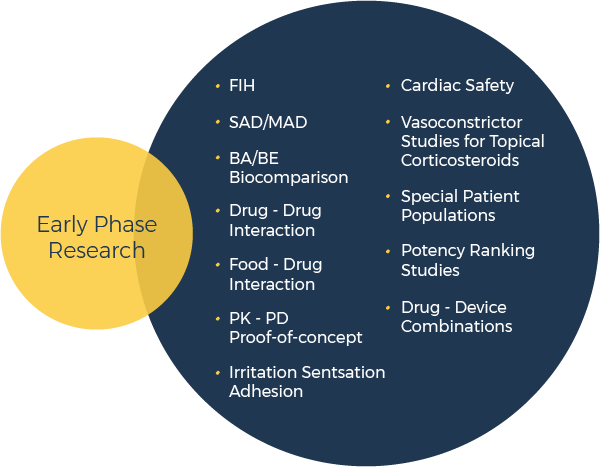
Novum’s Clinical Pharmacology Research Units in Las Vegas, NV and Toronto, Canada, draw upon decades of experience to handle virtually every dosage form and study type that can be conducted in healthy volunteers.
Novum’s Clinical Trial Management Group handles all Early Clinical Development studies that must be conducted in patients.
Novum’s Expertise
FIH | SAD/MAD | PK/PD | Bioequivalence | DDI | Drug-Device | Human Factor Studies | Transdermal Full Services
Novum’s Clinical Pharmacology Unit Experience
Respiratory Products (MDI, DPI, and Nasal Sprays) | Transdermals | Oral Dosage Forms | Biologics | Locally Acting Products | Natural Source Products
Novum’s Clinical Trial Management Group’s Patient-Based Experience
Oncology | Oncohematology | Neurology | Respiratory | Dermatology | Women’s Health | Autoimmune