Novum provides a comprehensive range of services in managing your clinical programs across Phase I-IV. Early Phase Studies in our two Clinical Pharmacology Units, multicenter Clinical Endpoint Studies, and Bioanalytical services covering large and small molecules.
Hematology/oncology, CNS/psych, dermatology, respiratory, women’s health, nephrology, immunology, and cardiovascular are just a few of our specializations.
Guided by a senior management team with global expertise in science, operations, and quality assurance, Novum offers the highest levels of integrity and reliability.
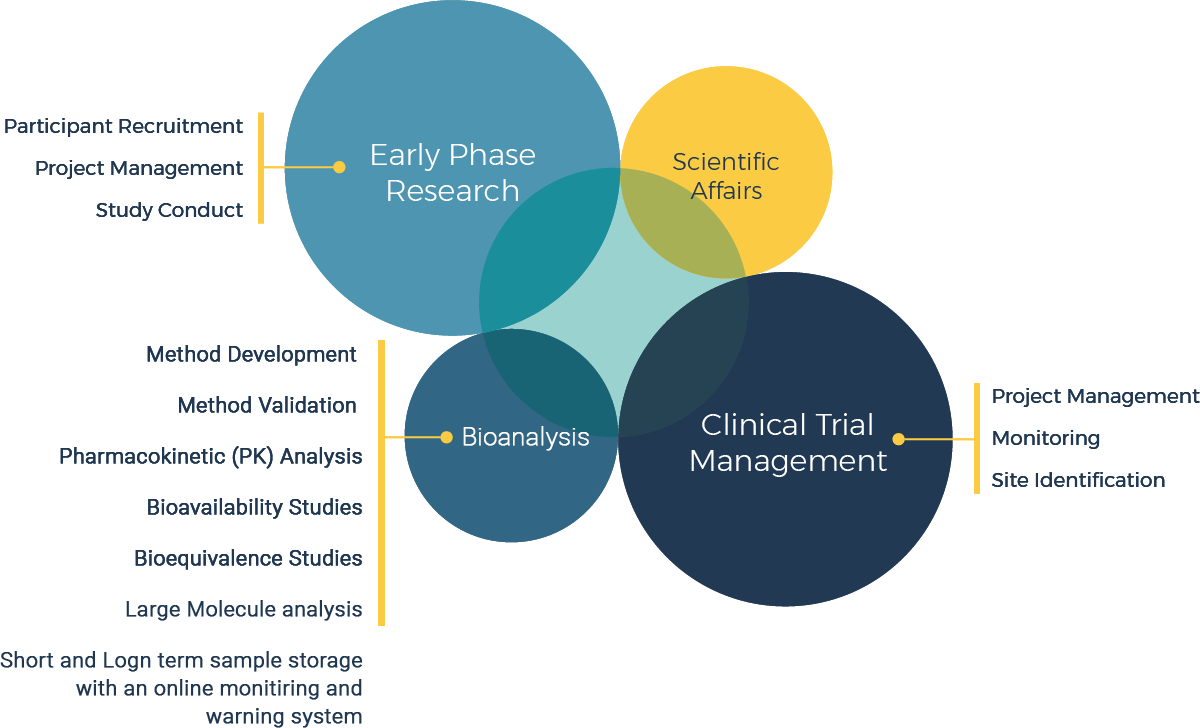
By applying scientific acumen to each step of every study and maintaining the highest quality and safety standards, Novum has a proven track record serving a diverse clientele of all sizes from around the world.
Contact us today to find out how we can help you with your drug development needs.

